When applying for package or label approval, it is important to understand the difference between packages and labels. A package is a physical structure that holds and protects the product. The label is all of the text, graphics, pictures, and logos printed on or affixed to the package.

PACKAGE – the Mylar bag on its own without any design or text elements

LABEL – includes the printed design, the logo, and all the text anywhere on package
PACKAGING
General Requirements
Each marijuana and hemp item must be packaged in a container that conforms to the rules found in OAR 845-025-7000 through 845-025-7190. A "container" is defined as a sealed, hard or soft-bodied receptacle in which a marijuana or hemp item is placed and any outer receptacle intended to display a marijuana item for ultimate sale to a consumer. This definition refers to any package or receptacle that holds a marijuana or hemp item and all outer packages used to display the marijuana or hemp item. For example, if a licensee packages an extract in a small round jar and then puts that jar into a cardboard box, both the jar and the box will be considered containers.

Small round jar placed inside of a cardboard box. Both the jar and the box are considered “containers.”
OAR 845-025-7160 requires all licensees and registrants who package marijuana or hemp items for ultimate sale to a consumer, patient, or designated primary caregiver to get packages and labels approved through the OLCC pre-approval process (see Pre-approval Process Section).1 “Ultimate sale” means the final sale from a retail location or dispensary to a consumer, patient, or designated primary caregiver. Packages and labels must be approved before any marijuana or hemp item is sold, offered for sale, or transferred between licensees or to a consumer, patient, or caregiver, unless subject to an exception.

OAR 845-025-7160 requires all licensees and registrants who package marijuana or hemp items for ultimate sale to a consumer, patient, or designated primary caregiver to get packages and labels approved through the OLCC pre-approval process (see Pre-approval Process Section).1 “Ultimate sale” means the final sale from a retail location or dispensary to a consumer, patient, or designated primary caregiver. Packages and labels must be approved before any marijuana or hemp item is sold, offered for sale, or transferred between licensees or to a consumer, patient, or caregiver, unless subject to an exception.
Packages must protect the marijuana and hemp items they hold. Packages and containers that hold marijuana or hemp items must protect those items from contamination and must not expose the marijuana or hemp item to any toxic or harmful substance.

Packages cannot contain untruthful or misleading statements. A false or misleading statement is one that is either not true or a statement that implies something about the product or package that is not true. For example, a label making a claim that the product in the package treats or cures a disease, when there is no significant scientific information to support that claim, would be a misleading statement. Similarly, labeling a product or its ingredients as "organic" when the product has not been properly certified would also be a misleading statement. See the sections on Organic and Health Claims for more information.
Packages must be labeled as required by OAR 845-025-7000 to 845-025-7190. A package must contain a complete and compliant label. The label can be printed directly on the package, securely affixed to the package, or both. All label information must comply with the labeling rule requirements.
Marijuana and hemp items cannot be packaged in a manner that is attractive to minors. Any of the following items would be considered “attractive to minors:”
-
Cartoons;
-
Designs, brands, or names that resemble a non-cannabis product that is typically marketed to minors;
-
Symbols or celebrities that are commonly used to market products to minors would be considered “attractive to minors;”
-
Images of minors; and
-
Words that refer to products that are commonly associated with minors or marketed by minors.
A "cartoon” is defined in rule as any drawing or depiction of an object, person, animal, creature or any similar caricature that:
-
Uses comically-exaggerated features;
-
Attributes human characteristics to animals, plants or other objects; or
-
Attributes unnatural or extra-human abilities, such as imperviousness to pain or injury, X-ray vision, tunneling at very high speeds, or transformation (i.e. Superheroes).
Packages cannot appear similar to any consumer product typically marketed towards minors or use the same types of symbols or designs that are used to market products to minors. All marijuana and hemp items, except plants and seeds, must leave the retail store in a childresistant package. The marijuana or hemp item can either be packaged in a container that is childresistant or the item can be placed into a child-resistant exit package at the point of sale.
All marijuana and hemp items, except plants and seeds, must leave the retail store in a childresistant package. The marijuana or hemp item can either be packaged in a container that is childresistant or the item can be placed into a child-resistant exit package at the point of sale.
 IMPORTANT! In order for a package to be considered child-resistant, the
package must be tested and certified as meeting the federal standards set out in
16 CFR 1700 by a qualified, third-party testing firm.
IMPORTANT! In order for a package to be considered child-resistant, the
package must be tested and certified as meeting the federal standards set out in
16 CFR 1700 by a qualified, third-party testing firm.
Child-resistant packages come in two forms: (1) single-use and (2) resealable, continually childresistant. A single-use, child-resistant package is one that meets the child-resistance standard for a single use and is child resistant until it is opened. A resealable, continually child-resistant package is one that is capable of being resealed after being opened and maintains child-resistant properties throughout the life of the product. See Child-Resistant Packaging section.
Resealable and Continually Child-Resistant Vs Single-Use Child-Resistant

This vial is an example of a resealable and continually, childresistant package because it is childresistant every time it is properly closed.

This Mylar bag is an example of a single-use package because once it is opened, it is no longer child-resistant.
The type of child-resistant packaging required depends on the marijuana or hemp item being sold in the container. Immature marijuana plants and seeds do not require the use of child-resistant packages. If the item is usable marijuana, the item can be packaged in a single-use, child-resistant package. All other items must be packaged in a resealable, continually child-resistant package. The item may be placed directly in a container that meets the child-resistance standard outlined in the table below or a non-child-resistant container may be placed in an approved child-resistant, exit package. See Table Below.
| Type of Packaging Required | Re-sealable & ChildResistant throughout Life of the Product | ngle-Use ChildResistant | Child-Resistant Packaging Not Required |
| Type of Marijuana Item Sold |
|
|
|
Child-Resistant Packaging

The term "child resistant" is defined in OAR 845-025-7000 as packaging that is designed or constructed to be significantly difficult for children under five years of age to open and not difficult for adults to use properly. Under OAR 845-025-7020, all marijuana and hemp items for sale to a consumer, patient, or designated primary caregiver, except for plants and seeds, must be packaged in a container that is child-resistant as certified by a qualified third party child-resistant package testing firm.
The standard for child-resistant packaging is set by the Consumer Product Safety Commission (CPSC). To determine whether a package meets the standard for child-resistance, a third-party testing firm follows the testing procedure found in 16 CFR 1700.20. If a package has been tested by a qualified firm, proof of certification must be provided to the OLCC before the OLCC can approve the package as meeting the childresistant standard.
The CPSC maintains a list of testing firms. A copy of that list can be found in the Child-Resistant Testing Firms section. The OLCC does not endorse or recommend any of the firms listed.
Exit Packaging

OAR 845-025-7000 defines “exit package” as “a sealed, child-resistant certified receptacle into which marijuana items already within a container are placed at the retail point of sale.” Exit packages can be used to add child resistance to a container that is not child resistant on its own. Because all marijuana and hemp items, except plants and seeds, must leave the dispensary or retail store in a child-resistant container, placing a non-child resistant container inside of an approved exit package will satisfy the child-resistant requirement. Marijuana items can be displayed in the store in non-child resistant packages but those packages must be placed in child-resistant exit packages at the point of sale. Additionally, multiple products can be placed in the same exit package at the point of sale.
Just like other types of packages, all exit packages must be approved through the OLCC Pre-approval Process. The fee for approval is $100 per package. Any package on the approved list may be used without additional approval. When certain changes are made to an approved package or label, the new package and / or label must be resubmitted to the OLCC. See the Pre-Approval Process section for more information.
Pursuant to OAR 845-025-7030(21), all exit packaging must contain a label that reads: "Keep out of the reach of children" in legible, typed font. This warning is the only required label information for an exit package. An exit package that has only this required warning printed on it2 without any additional text, graphics, logos, or pictures, would have a generic label that would not require OLCC label pre-approval. However, if the exit package contains any logos, pictures, graphics, or additional text not required by rule, the label is not generic and would need to be submitted for label pre-approval with an additional $100 fee.
The exit package may be provided by the producer, processor, or wholesaler that packaged the marijuana item for sale or the retail store or dispensary where the marijuana item is sold. Regardless who provides the exit package, it must be approved for use by the OLCC.
Retailer / Dispensary Responsibility
The retailer or dispensary is responsible for making sure that products that require a child-resistant exit package leave the store in an approved exit package. If the container holding the marijuana item is child resistant and on the OLCC approved list, it does not need an exit package. However, if the item is not in a child-resistant package, the retailer or dispensary is responsible for making sure that the marijuana item leaves the store in an OLCC-approved exit package. If the package has not been approved by the OLCC, it cannot be used.
Re-using Packaging
Only packaging that is resealable and continually child-resistant may be re-used. If a marijuana item is placed in a package that is being re-used, the old label or labels must be removed, and the package must have a new label or labels attached to it. Additionally, any packaging that is being re-used must be clean. The package cannot contaminate the marijuana or hemp item and must not expose the item to any toxic or deleterious substances. Exit packages may be reused as long as they are re-sealable, remain child resistant throughout the life of the product, and are in good working order.
LABELING
General Requirements All containers must be properly labeled. This includes any container that holds a marijuana or hemp item as well as any container used to display a marijuana or hemp item for sale. For information on small or tiny container labels, please read the Small Container Labeling section.
A label is any written, printed, or graphic matter affixed to, applied to, attached to, blown into, formed, molded into, embossed on, printed on, or appearing upon or adjacent to a package containing a marijuana or hemp item for purposes of branding, decorating, identifying, or giving any information with respect to the item or to the contents of the package. If a package contains multiple stickers or has some information printed directly on the container and the rest of the information on a sticker, all of the information is considered part of one label.
Each marijuana product type has specific requirements that must be included on the label. The label requirements for each product type can be found in OAR 845-025-7000 through 845-025-7190. All labeling requirements outlined in the rules are considered required information that must be included on the label. Failure to include required information on a label may result in the denial of a label application or a license violation. For a checklist of the specific requirements for each product type, go to the Label Checklist Section. If a container is too small to fit all of the required information, a small or tiny container label may be utilized. See Small Container Labeling section. Regardless of the product type, all labels must follow the same general requirements.
All the required information on a label must:
1. Be in typed, legible font that is at least 1/16th of an inch in height based on the uppercase “K,” although the font can be larger;
2. Be in a font that is easy to read and contrasts sufficiently with the background;
3. Be in English, but the information can be included in other languages;
4. Be unobstructed and conspicuous, meaning that all required information must be visible on the outside of the package; and
5. Be printed on or securely affixed to the package, meaning that the label will not fall off or be removed during transportation or normal use of the product.
Additionally, every label must contain:
1. A principal display panel as defined by OAR 845-025-7000. (See the Principal Display Panel Section for more information).
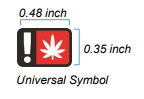
2. The universal symbol (at least 0.48 inches wide by 0.35 inches tall); and
3. All of the information required by rule for the specific product type (plant, seed, usable marijuana, edible, topical, concentrate, extract, or tincture).

This photo shows a package with two label panels. Both label panels are considered part of one label.
Non-required information can be in any font or size. Although there is a font size requirement for all required information, any additional information that is not required by rule may be in any font type and size as long as that text complies with the rest of the rules.
A package may have more than one label panel attached or affixed to it. Label information can be printed directly on the package, affixed to the package (i.e. with glue or as a sticker), or embossed into or printed directly on the package. For example, printing some required information directly onto a Mylar bag and including the rest of the required information as a sticker is compliant under the rules. Both the sticker and the information printed directly on the bag will be considered to be two parts of one label.
If your product falls into one or more categories that item must comply with the labeling requirements for both categories. For example, a concentrate that can also be consumed like an edible must have the labeling requirements for both concentrates and edibles, with the exception of the "DO NOT EAT" warning because the product is intended for human consumption and the "BE CAUTIOUS" warning if the effects of the product are customarily felt immediately.
Testing information for all laboratories and tests must be included on the label. If an item was tested by more than one lab or has more than one test analysis date associated with it, each lab and test analysis date must be included on the label. For example, if one lab tests for THC concentration and a different lab tests for pesticides, the information for both labs and tests must be included on the label. (See example of lab information on the right.) Similarly, if a first test fails and a subsequent re-test passes, the information for both tests must be included on the label.
Under OAR 845-025-7030 when listing the potency on a label, the amount of THC and CBD must be the value calculated by the laboratory that did the testing in accordance with OAR 333-064-0100. The potency value shall be expressed as an average of the samples taken and tested under OAR 333-007- 0360. A label may not have a THC value that exceeds the applicable maximum concentration limit. For more information about labeling THC and CBD, see the Potency section below
Label Prohibitions A label may not:
1. Contain any untruthful or misleading statements, including incorrectly using the term "organic" or making an unsubstantiated health claim;
2. Be attractive to minors, as defined in OAR 845-025-7000;
3. Specifically encourage the transportation of marijuana items across state lines;
4. Assert that marijuana items are safe because they are regulated by the Commission or because they have been tested by a certified laboratory or otherwise make claims that any government agency endorses or supports marijuana;
5. Make claims that recreational marijuana has curative or therapeutic effects;
6. Display consumption of marijuana items;
7. Contain material that encourages the use of marijuana because of its intoxicating effect; or
8. Contain material that encourages excessive or rapid consumption.
Elements of a Label
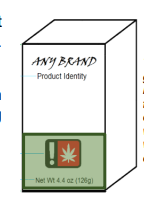
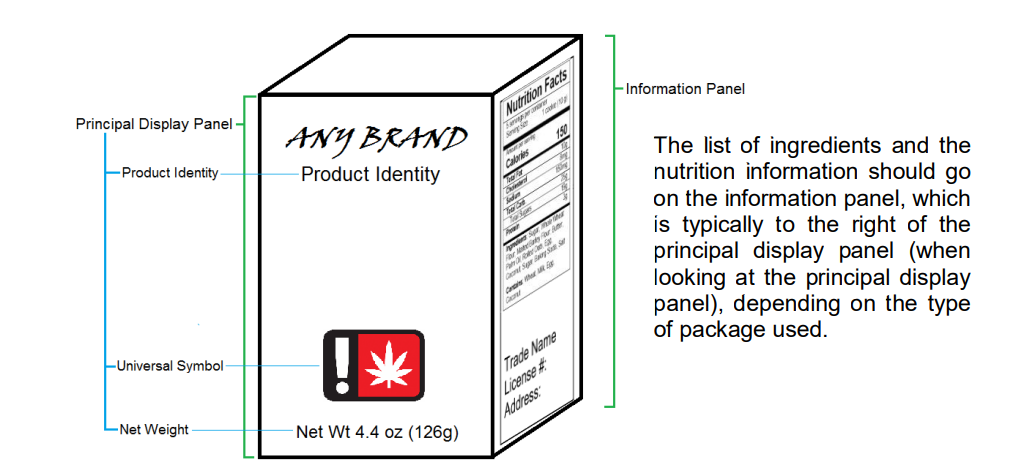
Principal Display Panel
The principal display panel is defined as the part of a label on a package or container that is most likely to be displayed, presented, shown or seen under customary conditions of display for sale or transfer, generally the front of the package that contains logos and branding. A package may have more than one principal display panel and, if so, all principal display panels must be properly labeled.
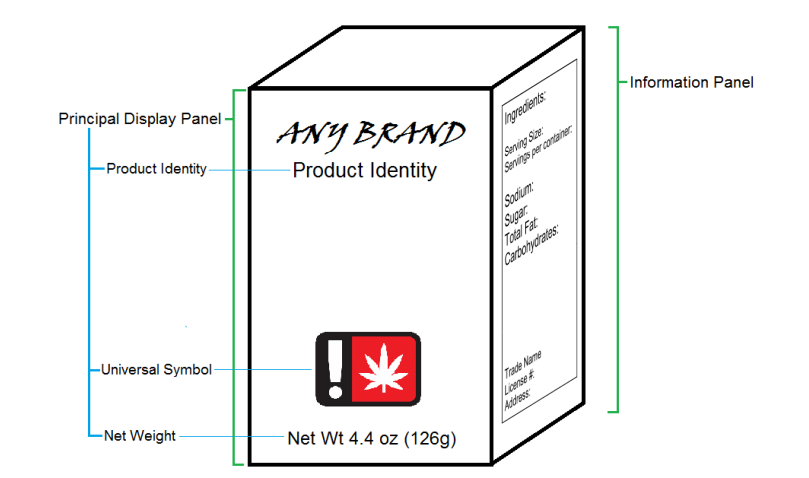
Example of a Package with two Principal Display Panels

In the above example, the package contains branding on the front and the top of the package. Both are considered principal display panels and both must be labeled properly
For most labels, three items must appear on the principal display panel:
(1) the universal or hemp symbol;
(2) the net weight or volume; and
(3) the product identity. All three items must be visible on the package at the same time.
Example of an Incorrectly Labeled Principal Display Panel
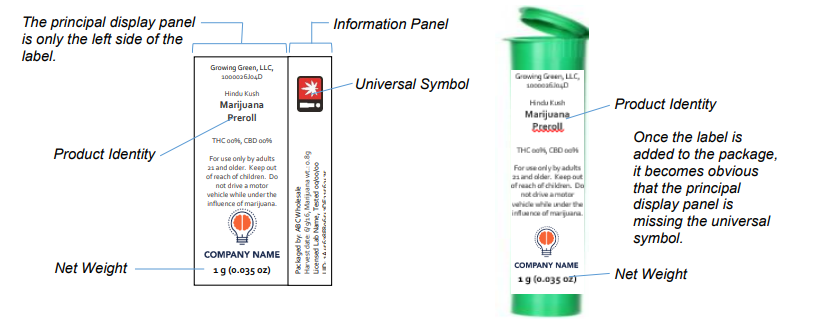
In the example above, the principal display panel is not labeled correctly. When the label is affixed to the package, only the product identity and net weight are visible on the principal display panel. To fix this label, the universal symbol must be moved so that the universal symbol is a part of the principal display panel.
Product Identity The product identity is the common or usual name of the product. This is a descriptive name for the product and not a fanciful name or the brand name of the product. For example, on a package of Starburst®, the name “Starburst” is the brand name of the candy, and the term “fruit chews” is the product identity

The product identity must:
-
Be in bold type,
-
Be in a size reasonably related to the most prominent printed matter on the principal display panel,
-
Be parallel to the base on which the package rests as it is designed and displayed; and
-
Clearly identify whether the item is derived from marijuana or hemp.
Net Weight or Volume (Net Quantity of Contents) The net quantity of contents means the amount of product being sold in the container. It can be expressed on the label as either the net weight or the net volume.
The net weight is the gross weight of the final product minus the weight of the packaging and is expressed on the label in both ounces and grams (or milligrams for weights under one gram). A label should include the net weight if the product is a solid, semi-solid, or viscous product. A standard net weight declaration looks as follows: Net wt 1.0 g (0.035 oz)
The net weight is the weight of the final product. For pre-rolls, the net weight is the weight of the finished pre-roll, which includes the dried marijuana leaves and flowers, the rolling paper, and the filter or tip.
The net volume is the fluid measure of a liquid product expressed as milliliters and fluid ounces (fluid ounces are different than ounces). A label should include the net volume if the product is a liquid.
Displaying Net Weight or Volume
The net quantity of contents provided on the principal display panel must be the average quantity of contents in all of the packages in the batch. The net quantity declaration must be:
A distinct item separated from other printed label information on all sides by at least a space equal to the height of the lettering used in the declaration;
-
In bold type;
-
In the bottom 30 % of the principal display panel;
-
In lines generally parallel with the base of the container; and
-
Listed in both the US Customary Units and the International System of Units (SI Units).
The shaded
green area is the
bottom 30% of
the principal
display panel
where the net
weight should be
displayed.
| US Customary | SI Units |
|
Weight (dry) displayed in ounces Volume (liquid) displayed in fluid ounces |
Weight (dry) displayed in grams or milligrams Volume (liquid) displayed in milliliters |
The net quantity of contents should be displayed as a number between 1 and 1000. When choosing a unit, use the following examples. If using a decimal, use no more than three decimal places.
Examples:
500 mg, not 0.5 g
1.96 g, not 1960 mg
750 mL, not 0.75 L
Net weight or volume should not be expressed in mixed units.
Example:
1.5 g, not 1 g 500 mg
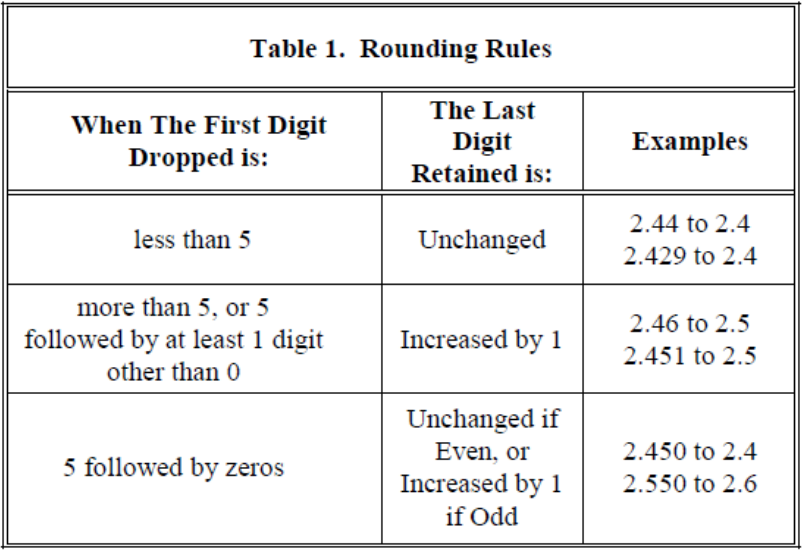
Rounding
Use Table 1 (above) from the NIST Handbook 130 (2015) to help with rounding the net quantity
Universal Symbol
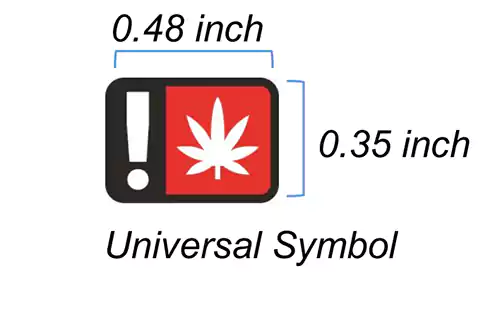
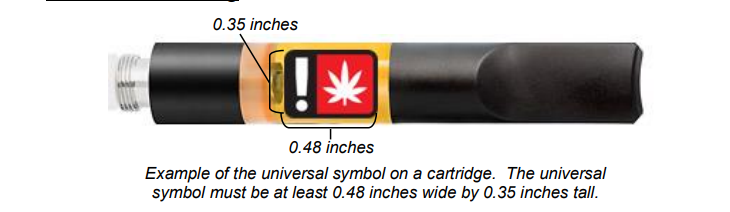
The universal symbol was created by the Oregon Health Authority and must be used on the label of a marijuana item that will ultimately be sold to a consumer. The universal symbol must be located on the principal display panel and must be at least 0.48 inches wide by 0.35 inches tall.
The universal symbol must be red, black, and white and cannot be changed from how it appears in the example provided. The universal symbol is required on all marijuana item labels. Additionally, licensees and registrants must receive OLCC approval for all labels prior to any sale to a consumer, patient, or caregiver. (See the Pre-Approval Process section for more information).

Universal Symbol
Hemp Symbol
The hemp symbol must be used in the place of the universal symbol when the product is made of hemp derived product. If an item contains both marijuana and hemp products, the item is considered a marijuana product and the label will not contain the hemp symbol. Hemp items have different labeling requirements than marijuana or mixed marijuana/hemp items. Please read OAR 845-025-7140 for more information.

Hemp Symbol
Medical Grade Symbol
The medical grade symbol was established by the Oregon Health Authority and made available to OLCC licensees. The medical grade symbol is a symbol that is used only by OLCC licensees that produce cannabinoid products, concentrates, or extracts that have a THC concentration that is above the recreational concentration limit.


Principal display panel with medical grade symbol.
The medical grade symbol is used only on products sold at OLCC licensed retail stores. Products that contain a medical grade symbol can only be sold or transferred to a designated primary caregiver or patient, for use by a patient. Licensees who want to produce medical grade products must follow the requirements set out in OAR 845-025-3300, as well as the rest of the rules. Any licensee that wants to process or sell medical grade products must register with the OLCC by completing the form found here:
IMPORTANT! The medical grade symbol is used in addition to the universal symbol - both symbols are required. The medical grade symbol must appear on the principal display panel and be at least 0.35 inches in diameter. Any medical grade product should contain the warning “For use by OMMP patients only” rather than the recreational warning, “For use only by adults 21 and older.”
RECREATIONAL THC CONCENTRATION
| Marijuana Item Type | Maximum THC per serving | Maximum THC per container |
| Edibles | 5 mg | 50 mg |
| Topicals | N/A | 6% |
| Tinctures | N/A | 1,000 mg |
| Capsules | 10 mg | 100 mg |
| Concentrates or Extracts | N/A | 1,000 mg |
| Other Products | N/A | 1,000 mg |
MEDICAL THC CONCENTRATION
| Marijuana Item Type | Maximum THC per serving | Maximum THC per container |
| Edibles | N/A | 100 mg |
| Topicals | N/A | 6% |
| Tinctures | N/A | 4,000 mg |
| Capsules | 100 mg | 4,000 mg |
| Suppositories | 100 mg | 4,000 mg |
| Transdermal Patches | 100 mg | 4,000 mg |
| Concentrates or Extracts | N/A | 4,000 mg |
| Other Products | N/A | 4,000 mg |
Potency
The THC concentration on a label cannot exceed the maximum concentration limit as determined by
the Retail and Medical Concentration Limit Tables. A marijuana item labeled with a THC concentration
in excess of the amount listed in the Retail THC Concentration Limit Table will be considered mislabeled
and will need to be fixed before it can be sold or transferred.
The THC and CBD amounts required to be on a label must be the value calculated by the laboratory that did the testing in accordance with OAR 333-064-0100. The potency value shall be expressed as an average of the samples taken and tested under OAR 333-007-0360.
A label may provide a target potency amount on the principal display panel as long as:
-
The target amount listed is within 10% of the THC and CBD amounts calculated by the lab; and
-
The actual THC and CBD amounts calculated by the laboratory are also provided elsewhere on the label.
Other Labeling Requirements
UID Number
All licensees must use the unique identification (UID) number provided by Metrc on the label. This number is the 24-digit Metrc tag number. The UID number on the label should be the number associated with the product at the time the product is packaged and labeled.
Activation Time
Activation time is the amount of time it is likely to take for an individual to begin to feel the effects of ingesting, inhaling, or using a marijuana item. Activation time may be expressed in words or through a pictogram. If a user will begin to feel the effects right away, the activation time can be listed as immediate. If the product has a delayed reaction, the licensee or registrant must determine what the activation time is for their particular product. To show activation time on a label, you may simply state, “Activation Time: 30 minutes” or you may use a pictogram (see example on right), as long as the pictogram is clear and easily understood.

Pictogram Example.
Business or Trade Name and License or Registration Number
All labels require the business or trade name and the license number (if you are an OLCC license) or a registration number (if you are an OHA registrant). These two pieces of information should be listed together on the label. Providing the business or trade name as part of a logo or other branding is not sufficient to meet this requirement. The label must be clearly marked with the identifying information for the licensee or registrant responsible for the product.
Serving Size and Measuring Devices
Some labels must provide a serving size and the number of servings in the container. The serving size is the amount of product that is suggested for a consumer to use. The serving size is NOT the potency of the product. Instead the serving size should explain to the consumer the amount of product he or she should use to reach a specific potency. The serving size is determined by the processor, but the label must follow the rules regarding concentration limits. For example, if product being sold was a cookie that contained 10 mg THC, the serving size could be ½ of the cookie because the concentration limit per serving for an edible is 5 mg THC. In this example the label would say, “Serving Size: ½ cookie, Number of Servings per Container: 2.”
If the product being sold was a concentrate, the processor would decide how much a consumer should consume at one time and list that amount on the label. For example if the container held one gram of concentrate, the processor could decide that the serving size should be 0.03 grams or 30 milligrams. The label would say, “Serving Size: 30 milligrams or an amount equal to a grain of rice, Number of Servings per Container: 33.”
Edible products must either be scored or if the product cannot be scored, the package must contain a measuring device that measures single servings or the package must clearly enable a person to determine when a single serving has been consumed. Measuring devices must be large enough for the consumer to use as a reliable guide for measuring single servings. A small pictogram on a label will not be sufficient.
Labeling Small Containers
Small Container Labels
All containers that hold a marijuana item must be properly labeled. Under OAR 845-025-7030(11), if the container holding the marijuana or hemp item is too small to fit all of the required label information, a licensee or registrant may put at a minimum the following information on a label that is securely affixed to the small container:
-
Principal display panel that includes the net weight, universal symbol, and product identity;
-
Licensee or registrant business/trade name;
-
License or registrant number;
-
UID number;
-
Concentration of THC and CBD; and
-
Required warnings.
-
For a retail marijuana item or industrial hemp commodity or product, the following warning is required on the label: “For use only by adults 21 and older. Keep out of reach of children.”
-
For a medical marijuana item, the following warning is required on the label: “For use by OMMP patients only. Keep out of reach of children.”
The remaining required information must be included on an outer package or container or on a hangtag attached to the small container. If an outer package is used, all of the information required by rule must be on the outer container, even if some of the information is already included on the inner container. In other words, if a small container is packaged inside a larger container, the outer container must have a full label. If a hangtag is used, the hangtag must be securely attached to the small container and contain the rest of the required information that is not already listed on the small container label (for example: lab name, test date, serving size, etc.). Small containers can utilize accordion or peel back labels but the information required on a small container label must be visible on the outside of the accordion or peel back label.
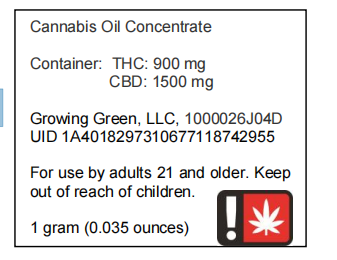
Example of a small container label for a concentrate.
Small Container Label Example
In this example, the small tincture bottle is too small to fit a full label. The small bottle must have at least the information required by the small container label rule printed directly on or affixed to the small container. The remaining information must either go on a leaflet, hangtag, or outer container. Please see the examples below.


Hangtag for small container

Full Label on Bag (not to scale)
Tiny Container Labels Under OAR 845-025-7030(12), a marijuana or hemp item that is packaged in a container that has a complete surface area available for applying a label that is less than 2 inches squared may have a label printed on or affixed to the container that includes at a minimum the following information:
A principal display panel with the universal symbol and product identity;
-
UID number;
-
Concentration or amount of THC and CBD in the container;
-
Licensee or registrant business/trade name;
-
License or registrant number; and
-
A warning that reads: “Keep out of reach of children.”
Similar to a small container label, the remaining required label information must be included on a hangtag attached to the tiny container or the tiny container can be packaged inside of an outer container that contains a full and compliant label.

Tiny Container Label Example
All cartridges and vaporizing devices containing a cannabinoid concentrate or extract or product intended for use with an inhalant delivery system must be labeled with the universal symbol. The universal symbol must be 0.48 inches wide by 0.35 inches tall. The universal symbol can either be printed directly on the cartridge or it can be attached to the cartridge as a sticker. Cartridges are not required to have a small container label.
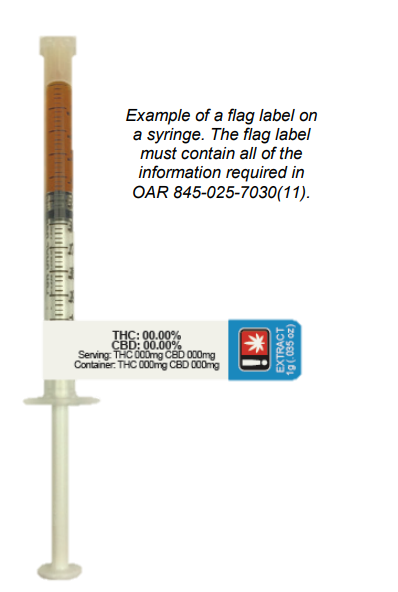
Syringes do not fall under this rule and must have a small container label attached to them. In order to fit all of the required information, a flag label (see example on right) may be used. For more information about small container labels, please see the Small Container Labeling section.
Edible Labeling
For cannabinoid edibles, it is required that the following information be placed on the label:
1. List of all ingredients in descending order of predominance by weight or volume used to process the cannabinoid edible. The list of ingredients must include any substance used in processing, preparing, manufacturing, packaging, or holding the cannabinoid product that is present in the final product, including any cooking or release spray. The list of ingredients must correctly identify the type of marijuana item or industrial hemp ingredient used to make the product.
This includes all ingredients and sub ingredients. For example, in a chocolate chip cookie recipe, the ingredient list may be as follows: Ingredients: Enriched Flour, Brown Sugar, Chocolate Chips, Cottonseed Oil, Baking Soda, Salt.
However, both enriched flour and chocolate chips are composed of other sub-ingredients. For this example, the chocolate chips are made of cane sugar, chocolate liquor, cocoa butter, milkfat, and soy lecithin and the enriched flour is made of wheat flour, malted barley flour, niacin, iron, thiamin mononitrate, riboflavin, and folic acid. Because these two ingredients have ingredients of their own, you must list all of the ingredients and sub-ingredients in one of two ways:
First, you can list the names of the ingredients and then list any sub ingredients in parenthesis.
Ingredients: Enriched Flour (Wheat Flour, Malted Barley Flour, Niacin, Iron, Thiamin Mononitrate, Riboflavin, Folic Acid), Brown Sugar, Chocolate Chips (Cane Sugar, Chocolate Liquor, Cocoa Butter, Milkfat, Soy Lecithin), Cottonseed Oil, Baking Soda, Salt.
Second, you could list out each ingredient in descending order of predominance by weight or volume.
Ingredients: Wheat Flour, Malted Barley Flour, Niacin, Iron, Thiamin Mononitrate, Riboflavin, Folic Acid, Brown Sugar, Cane Sugar, Chocolate Liquor, Cocoa Butter, Milkfat, Soy Lecithin, Baking Soda, Salt.
2. The amount of calories, sodium, protein, added sugars, cholesterol, total carbohydrates, and total fat per serving. A cannabinoid edible shall use one of the nutrition information formats provided by the Commission to display the amount of calories, sodium, protein, added sugars, cholesterol, total carbohydrates, and total fat per serving, the serving size and number of servings per container, and the list of ingredients and potential allergens.
Even if the amount per serving is zero, it must still be listed on the label.
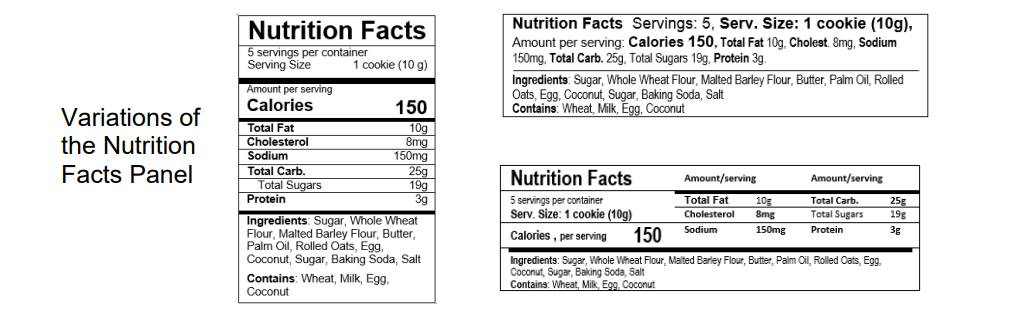
The rules do not require a specific type of analysis to determine the nutrient amounts displayed in the Nutrition Facts Panel but you may use one of the following methods to determine those values:
A. Database analysis. Using a list of ingredients and specific processing information for your product, you can use a food ingredient database to determine the specific nutrient amounts for your product. This method may be a better predictor of nutrient values across multiple batches versus a single laboratory test from one batch. However, how the analysis is performed affects the validity of the results. You must be extremely detailoriented and have a general knowledge of food and nutrient values, be able to understand and account for processing changes, and be able to keep detailed records.
B. Lab analysis. A lab can determine the nutrient values for the sample submitted to the lab. The results will only be specific to the sample tested so you may want to consider testing your product throughout the year to get the most accurate results. Lab analysis may be more beneficial if you are using unique ingredients that do not have nutrient information available or when the specific process you are using to make the edible is going to change the nutrient composition of the product in an unpredictable way.
C. A combination of database and lab analysis. You can verify a claim or cross check results using both methods.
IMPORTANT! You do not need to submit your nutrient analysis to the OLCC but a licensee or registrant must have documentation that demonstrates the validity of the calculations and must make that documentation available to the Commission or the Authority upon request.
3. If the edible is perishable, a statement that the edible must be refrigerated or kept frozen. If the edible is not perishable, no statement is needed.
4. List of potential major food allergens.
A licensee or registrant must list major food allergens on the label if the edible contains: Milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, or soybeans as an ingredient; or
Any ingredient that contains protein derived from: milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, or soybeans.
When labeling allergens, always use the specific food name for nuts, fish or crustacean shellfish and not the category of allergen. For example, use the word “almonds” instead of “tree nuts” in the Contains statement. Licensees and registrants must label major food allergens in one of two ways.
The first option is to include the name of the food source in parenthesis following the common or usual name of the major food allergen in the list of ingredients whenever the name of the food source of the major allergen does not appear elsewhere in the ingredient statement.
For example: Ingredients: Enriched flour (wheat flour, malted barley, niacin, reduced iron, thiamin mononitrate, riboflavin, folic acid), sugar, partially hydrogenated soybean oil, and/or cottonseed oil, high fructose corn syrup, whey (milk), pecans, eggs, vanilla, natural and artificial flavoring) salt, leavening (sodium acid pyrophosphate, monocalcium phosphate), lecithin (soy), mono-and diglycerides (emulsifier)
In the example above, the major food allergens are in bold to highlight their location. However, the allergens do not need to be in bold on an edible label.
The second option is to use the word "Contains" followed by the name of the food source from which the major food allergen is derived, immediately after or adjacent to the list of ingredients, in a font size that is the same font size used for the list of ingredients.
For example, after the list of ingredients, the following statement would appear: Contains Wheat, Milk, Pecans, Egg, and Soy
Gluten-Free
Gluten is the protein that occurs naturally in wheat, rye, barley, and crossbreeds of these grains. Although certain grains may contain gluten, some grains can be made gluten-free. An ingredient that has been derived from a gluten-containing grain can be labeled as "gluten-free" if it has been processed to remove the gluten and use of that ingredient results in the presence of less than 20 parts per million (ppm) gluten in the food. The "gluten-free" claim is a voluntary one, however, licensees and registrants who decide to use this term are responsible for using the claim in a truthful and not misleading manner, and for complying with the requirements established by the U.S. Food and Drug Administration.

Gluten-free means that the food either is inherently gluten free or does not contain an ingredient that is: (1) a gluten-containing grain (e.g. Spelt wheat); (2) derived from a gluten-containing grain that has not been processed to remove gluten (e.g. Wheat flour); or (3) derived from a gluten-containing grain that has been processed to remove gluten (e.g. Wheat starch), if the use of that ingredient results in the presence of 20 parts per million (ppm) or more gluten in the food. Any presence of gluten in the food must be less than 20 ppm.
False or Misleading Claims
Organic
Licensees and registrants that want to label their products as organic must follow strict requirements. First, if a licensee or registrant wants to make a claim that a product or its ingredients are organic, the product or certain ingredients need to be certified as organic. If it is not certified, the licensee or registrant cannot make any organic claim on the principal display panel or use the USDA organic seal anywhere on the package. Doing so will be considered misleading and could result in a denial of the label approval request. Second, licensees and registrants cannot use the word “organic” or any variation of the word in a way that implies the product is organic, the product has been certified organic, or that the marijuana in the product is organic. This means the logo and branding on a principal display panel or elsewhere on the label cannot use the word organic. To learn more about organic certification, please contact the Oregon Department of Agriculture at 503.986.4550.

"Made with organic ***" statement Licensees and registrants that want to label their products with the "Made with organic ***" statement must contain at least 70 percent certified organic ingredients (not including salt or water). These products may contain up to 30 percent of allowed non-organic ingredients. (See National list of Allowed and Prohibited Substances) All ingredients must be produced without GMOs or other prohibited substances and the product must be certified. If a product meets these requirements, its label may include a statement such as "made with organic wheat" that lists the specific organic products. The generic statement, "made with organic ingredients" is not allowed. The organic ingredients also must be identified in the ingredient list. Additionally, the label must identify the USDA-accredited certifying agent on the information panel.

Specific Ingredient Listings If the product contains less than 70 percent organic contents, the specific organic ingredients may be listed in the ingredient statement. You may only, on the information panel, identify the certified organic ingredients as organic and the percentage of organic ingredients. Licensees and registrants cannot include the USDA organic seal anywhere or use the word "organic" on the principal display panel. To learn more about the USDA Organic Program, check out the USDA Organic website:https://www.usda.gov/topics/organic
Health Claims
Health claims describe a relationship between a substance and a reduced risk of a disease or healthrelated condition. OAR 845-025-7030 prohibits the use of a health claim that is not supported by the totality of publicly available scientific evidence (including evidence from well-designed studies conducted in a manner which is consistent with generally recognized scientific procedures and principles), and for which there is significant scientific agreement, among experts qualified by scientific training and experience to evaluate such claims. A statement claiming that the product or an ingredient in the product can cure, mitigate, or treat any disease or health-related condition cannot be made or implied. Any statement that makes such a claim would be considered a misleading statement and could lead to a denial of a label application.
Strain Names The label cannot contain any words that refer to products that are commonly associated with minors, marketed by minors, or any names that are false or misleading. To help clarify which strain names are not allowed on labels or advertising, please look at the categories of names below and the examples under each category. This is not an exhaustive list of prohibited strain names but a few examples of what is NOT allowed.
First Category - Names of children’s toys or any character or other item in a children’s book, TV show, or movie. The following names are examples of the types of names that would be prohibited:
-
Incredible Hulk
-
Ewok
-
Optimus Prime
-
Light Saber
Second Category - Food Products Marketed to or by Children. The following names are examples of the types of names that would be prohibited:
Any Girl Scout Cookie - Thin Mints, Dosidos, etc.
-
Frosted Flakes
-
Lucky Charms
-
Skittles
Third Category - False or Misleading. The following names are examples of the types of names that would be prohibited:
-
Green Crack
-
Opium
-
Special K